Are you a Microbe Master? Take Our 8-Question Quiz and Start Brewing Today!

Kombucha is a living symbiosis of bacteria and yeast that’s been brewed and shared in homes for generations. A culture with that kind of staying power has a few built-in “defense systems” that help it thrive in a world full of microbes.
In Kombucha, the three biggest protection mechanisms are:
This page is about pH, the invisible shield that helps your brew stay in the safe lane.

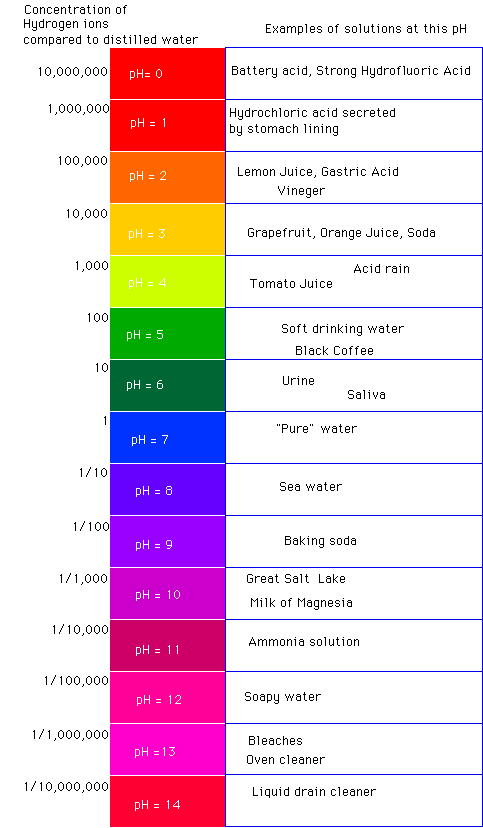
pH is a measurement of how acidic or alkaline a liquid is.
The scale is logarithmic, meaning each whole number change is a big jump in acidity or alkalinity (not a cute little step).
Kombucha is supposed to be acidic. That’s not a flaw, that’s the feature.
Think of low pH as a chemical force field.
Kombucha’s “native” bacteria and yeast thrive in acidic conditions, while many unwanted microbes struggle to gain a foothold there. In other words:
Proper acidification = fewer opportunities for invaders.
This is why starter tea matters, why fermentation temperature matters, and why Kombucha is typically more resilient than people think when brewed with good basics and clean habits.
A properly brewed batch of Kombucha commonly lands somewhere around:
The key takeaway for homebrewers is less about chasing a “perfect number” and more about ensuring the brew acidifies early and continues trending in the right direction.
pH does not tell you if Kombucha is “done.”
It tells you whether the brew has become acidic enough to be protective.
“Done” is ultimately a taste decision: sweet → balanced → tart.
Not always. pH and acid content are related, but they’re not the same measurement.
Two liquids can have similar pH and taste wildly different because:
Translation: pH is a tool, not a flavor oracle. Your taste buds still run the kingdom.
For a healthy first fermentation, you generally want to see the brew acidify into the “protective range” relatively early in the process.
If your brew stays high-pH for too long, that’s when you’re more vulnerable to unwanted growth.
Most of the time, “slow acidification” comes down to:
👉 See also: Brewing Location Guide + How to Keep Kombucha Warm + Best Brewing Vessel Guide
One of fermentation’s superpowers – and the original purpose of fermentation thousands of years ago when it was discovered – is preservation. When Kombucha is properly acidified, it’s naturally hostile to many of the organisms that cause classic spoilage.
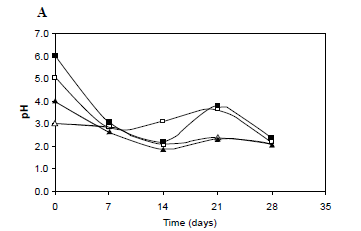
Researchers in Brazil found that Kombucha’s antimicrobial activities protected against E. coli, Salmonella typhi & M. canis and were found to be most effective after 28 days of fermentation (perfect for Continuous Brewers!). Their research also revealed the cyclical nature of the symbiosis. After about 14 days of steadily declining pH, the activity shifted and the pH gradually rose and peaked again at 21 days, then shifted lower again at 28 days (see graph to the right).
That doesn’t mean hygiene doesn’t matter. It absolutely does.
But it does mean Kombucha isn’t a fragile flower. It’s more like a tiny microbial fortress that prefers good housekeeping.
Pro tip: Once you’ve brewed a few batches, strips become a great “sanity check” instead of a daily ritual.
A pH meter is useful if you:
Basic meter workflow:
Kombucha Mamma Sez: You don’t have to test pH. But if it gives you peace of mind (or helps you teach your kids some kitchen science), I’m not here to stop you. 😉
Most well-fermented Kombucha typically ends up in the ~2.5–3.5 range, but the “right” pH depends on time, temperature, recipe, and how tart you like it.
No. pH confirms acidification, but “finished” is mainly a taste decision.
No. Many brewers never test. pH tools are most helpful for:
Yes. Sweetness and acidity can coexist, especially early on or in cooler ferments. Taste + time tell the full story.
Strips are easy and affordable. Meters are more precise and useful across multiple ferments. Either works.
pH is one of Kombucha’s oldest defenses, even if humans didn’t always have a number for it. When your brew acidifies properly, it becomes a safer, more stable fermentation environment, and your SCOBY gets to do what it does best: turn sweet tea into something alive, tangy, and uniquely yours.
Want the full “brew safety” picture (mold, temperatures, troubleshooting, the whole fortress plan)?
Head here next: Is Brewing Kombucha Safe? The Complete Guide to Kombucha Safety, Mold Prevention & Proper Fermentation.
How To Make Kombucha At Home (2026 Complete Guide)
What Is a SCOBY? 2026 Guide to Kombucha Cultures, Care & Creative Uses
Does Kombucha Contain Alcohol? The 2026 Guide To Kombucha & Alcohol (And How To Control It)
Top Uses for Kombucha Vinegar: A Practical 2026 Guide to Cleaning, Cooking, Gardening & More